Question 247Steam Plants - Assistant Engineer
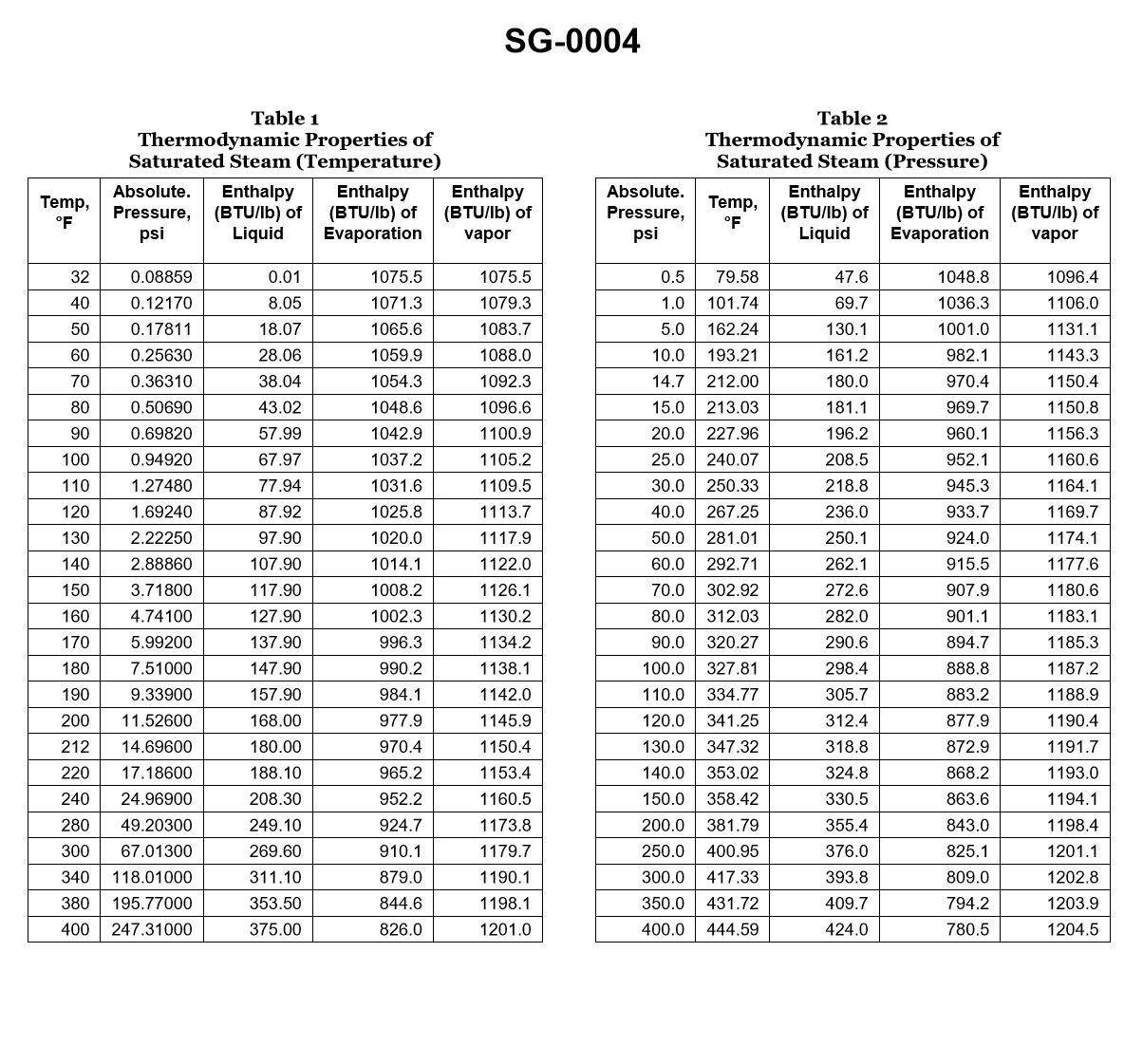
70% to passWith reference to the chart, if a boiler generates saturated steam at 385.3 psig, how much heat per pound was required to change the water into steam if the feed water temperature was initially 104.5°C? Illustration SG-0004
A96.85 BTU
B97.15 BTU
C1016.40 BTU
D1196.45 BTU
AI Explanation
The correct answer is C) 1016.40 BTU. To determine the heat required to change the water into steam, we need to calculate the enthalpy of vaporization (latent heat) at the given pressure of 385.3 psig. At this pressure, the saturated steam temperature is approximately 435°F (223.9°C). Using steam tables or appropriate thermodynamic calculations, the enthalpy of vaporization at this temperature is approximately 1016.40 BTU/lb. This is the amount of heat required to change the water into steam, given the initial feed water temperature of 104.5°C. The other options are incorrect because they do not accurately represent the enthalpy of vaporization at the specified pressure and temperature conditions.
Related Questions
Q67:A natural circulation water-tube boiler, with one or more water drums, would be ... Q521:On a ship equipped with a gravity type lube oil system, which of the conditions ... Q377:While your vessel is underway at normal speed, a steam drum safety valve develop... Q580:The main propulsion turbine can be damaged by _______________. Q548:Axial movement in a gear-type flexible coupling is provided for by _____________...
Ready to test your knowledge?
Take a Steam Plants - Assistant Engineer Practice ExamOfficial Resources