Question 231Steam Plants - 1st Asst/Chief
70% to passIf a boiler generates saturated steam at 125.3 psig, how much heat is required to change the water into steam if the feed water temperature is 240°F? Illustration SG-0004
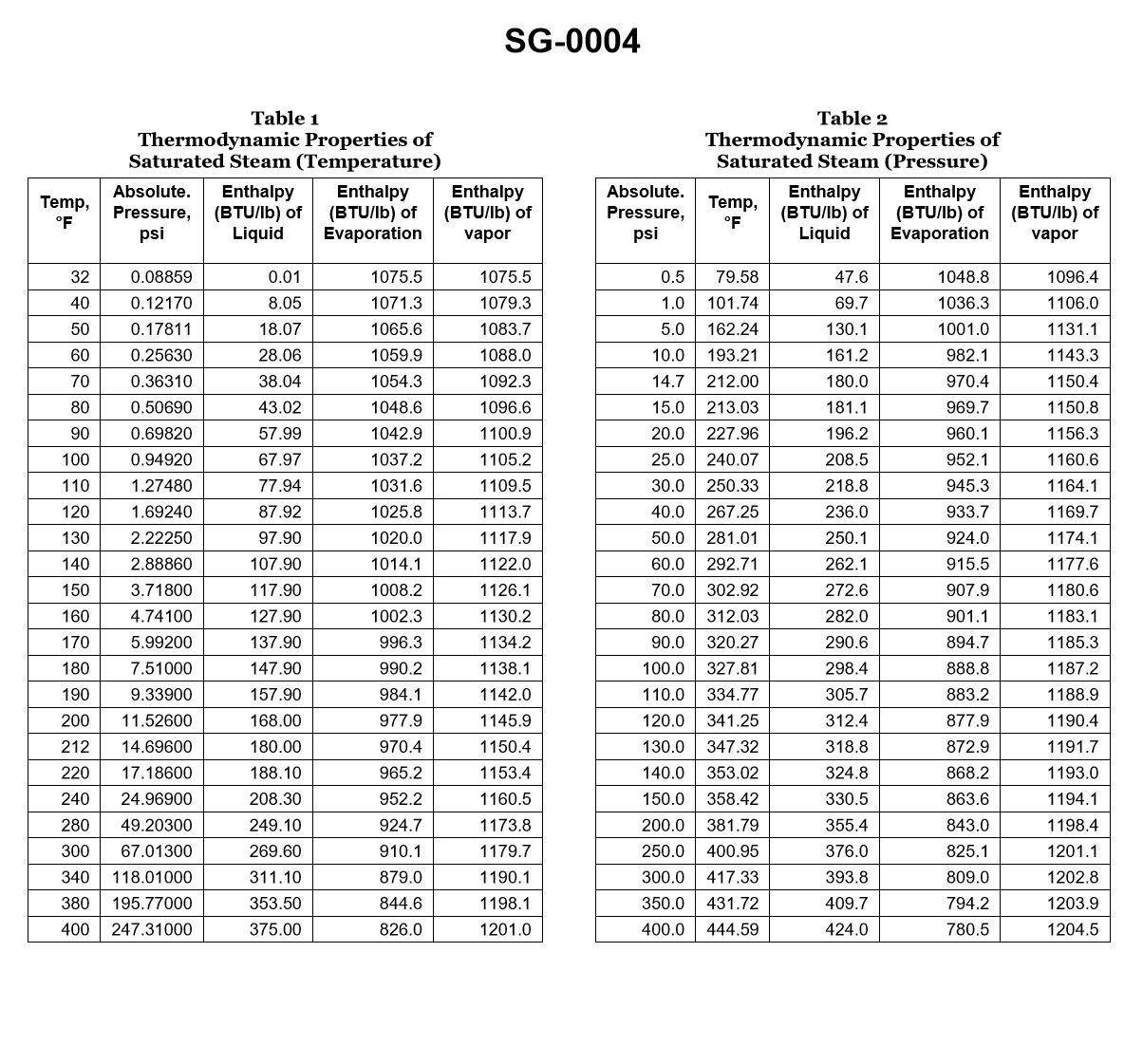
A30.5 Btu/lb
B116.5 Btu/lb
C582.7 Btu/lb
D983.4 Btu/lb
AI Explanation
The correct answer is D) 983.4 Btu/lb. The amount of heat required to change water into saturated steam at a given pressure is known as the latent heat of vaporization. At a pressure of 125.3 psig, the latent heat of vaporization for water is 983.4 Btu/lb. This value can be found in steam tables or other reference materials commonly used for marine engineering calculations. The other options are incorrect because they do not represent the correct latent heat of vaporization for the given pressure. Option A (30.5 Btu/lb) is too low, Option B (116.5 Btu/lb) is also too low, and Option C (582.7 Btu/lb) is significantly lower than the actual value.
Related Questions
Q99:In a steam assist fuel oil atomizer, the steam pressure is higher than the oil p... Q334:Excessive thrust bearing wear in a main propulsion turbine rotor should FIRST be... Q383:Which of the conditions listed could cause an oil flow sight glass, of a main tu... Q199:When the rate of heat transfer through tube walls is so reduced that the metal b... Q402:What is the FIRST thing that will happen if both the main and standby lube oil p...
Ready to test your knowledge?
Take a Steam Plants - 1st Asst/Chief Practice ExamOfficial Resources